The Latin cohaerere means to be together. The most important factor holding molecules together is the force of attraction between molecules. For example, since the attraction between liquid molecules is weak, the molecules slide over each other. There is a force of attraction between the molecules of the liquid and the molecules of other substances. The force of attraction between these molecules is called cohesion. The force of attraction between molecules of different types is called adhesion.
Examples from Daily Life
The cohesive force allows the liquid molecules to hold each other. For example, let’s consider a water drop, each particle forming the water drop is exerted by other particles by cohesion force. Due to the cohesion force, the particles can make translational movements, and they approach each other, reducing their surface area. Thus, the particles that make up the drop are gathered very close to each other and form a shape, and this shape is the sphere with the smallest surface area.
For example, raindrops take the shape of a sphere, water flows in the opposite direction in plants, and the water flowing from the hose flows straight like a rope. The surface tension that occurs due to the cohesive force of water causes some living things to stand on the water surface and even walk. Thanks to the cohesion force and adhesion force, the water can be transported in a column against gravity from the roots of the plants to the leaf tips.
What is adhesion?
Adhesion is the opposite of cohesion. It is the force of attraction between two different substances. It allows the liquid molecules to adhere to different surfaces. For example, when there is some water or coffee between the coffee cup and the plate we put on it, the plate and the cup stick together due to the adhesion force of the cup. Since the adhesion force is greater than the weight of the plate, the plate moves with the cup.
Cohesion and Adhesion
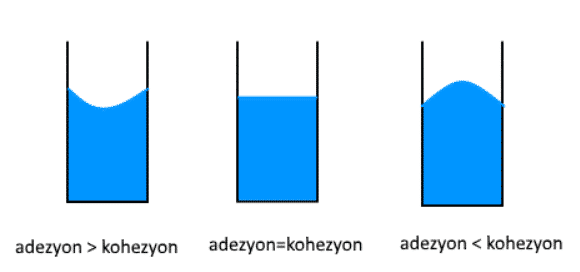
In some cases, one of the cohesion and adhesion forces may be greater than the other. When the cohesive force is greater than the adhesion force, the attraction force between the liquid molecules will be stronger, so the liquid in the container takes its convex shape. If the adhesion force is greater than the cohesive force, the liquid molecules will stick to the sides of the container more and take their concave shape. If the cohesion and adhesion forces are equal, the liquid surface in the container is flat.
Surface tension
For example, when we are swimming in the sea or in the pool, our hair is scattered in the water. When we come out of the water, our hair is collected downwards as a whole. In this case, there is the effect of cohesion, that is, the force of attraction that the liquids exert on each other. When the temperature of a liquid increases, its kinetic energy also increases, and accordingly, the particles move faster. At this speed, it causes a decrease in the force of attraction between the particles, and with the decrease of the force of attraction, the surface tension of the particles on the surface decreases. Factors such as substance type, temperature, pressure and whether the liquid is pure or not are some of the factors that affect the surface tension.